Crystallography based investigation of non-covalent interactions to design antidiabetic compounds
Crystallography based investigation of non-covalent interactions to design antidiabetic compounds Research Assistant,Lady Brabourne College, Kolkata (Sept 2022 - July 2023)
Supervised by Prof. Nayim Sepay
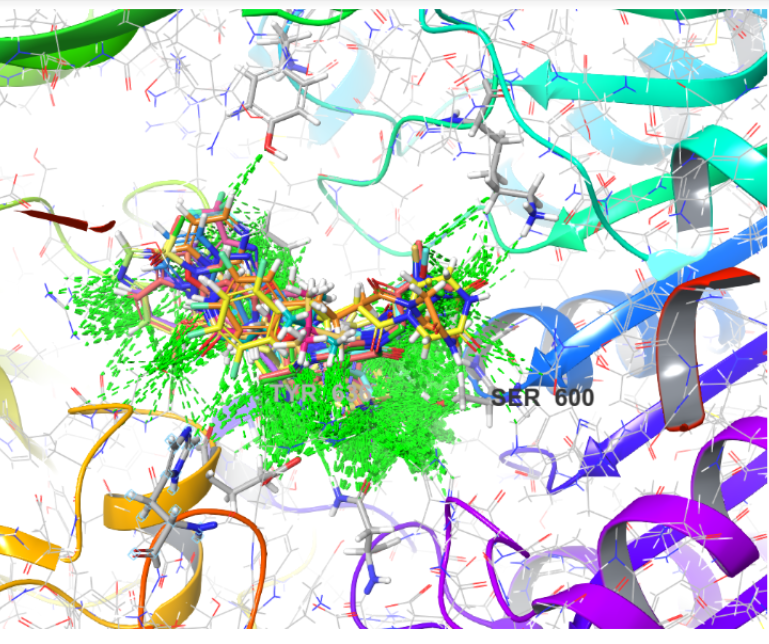 Protein molecules are a good target for the inhibition or promotion of biological processes. Different methods like QSAR and molecular docking have been developed to accurately design small binder molecules for target proteins. An alternative model has been developed wherein a statistical method is used to find the propensity of different non-covalent interactions between small molecules and amino acid residues of the protein. The results give hints as to the choice of substituents required at the SM to strongly bind to a protein.The statistical approach was to study various molecules of DPP4 bounded covalently or non- covalently with small molecules and through that, document the various functional groups present and the type of interactions shown in the active site with other ligands. By approaching via this method, it could be determined the amino acid residues partaking often to form bonds and gather what type of interactions occurred the most with the small molecules. Then with the data gathered, we could design a molecule that binds strongly with DPP4 with the effective binding interactions found. In order to identify efficacious molecules for inhibiting DPP-4, a quantitative analysis was conducted on 109 X-ray crystal structures of the DPP-4 protein that contained either covalently or non-covalently bound small molecules (SMs). The current investigation elucidates both ordinary and perceptive facets pertaining to protein inhibition.
Protein molecules are a good target for the inhibition or promotion of biological processes. Different methods like QSAR and molecular docking have been developed to accurately design small binder molecules for target proteins. An alternative model has been developed wherein a statistical method is used to find the propensity of different non-covalent interactions between small molecules and amino acid residues of the protein. The results give hints as to the choice of substituents required at the SM to strongly bind to a protein.The statistical approach was to study various molecules of DPP4 bounded covalently or non- covalently with small molecules and through that, document the various functional groups present and the type of interactions shown in the active site with other ligands. By approaching via this method, it could be determined the amino acid residues partaking often to form bonds and gather what type of interactions occurred the most with the small molecules. Then with the data gathered, we could design a molecule that binds strongly with DPP4 with the effective binding interactions found. In order to identify efficacious molecules for inhibiting DPP-4, a quantitative analysis was conducted on 109 X-ray crystal structures of the DPP-4 protein that contained either covalently or non-covalently bound small molecules (SMs). The current investigation elucidates both ordinary and perceptive facets pertaining to protein inhibition.
